Drug Discovery, Drug Design, and Drug Development are time-consuming as well as expensive, and financially risky but rewarding processes. The word “Drug” is derived from the Greek “Pharmacon” meaning “Drug” and the French word “Drogue” meaning “Dry Herb”. A drug is any chemical substance that when acts on the living body alters the physiological process and is used for the prevention, diagnosis, control, and treatment of disease. Difference between Drug and Medicine (Drug Product). Generally, a drug is available in the market by passing through 3 stages:
Drug Discovery >>> Drug Design >>>Drug Development
Table of Contents
Drug Design vs Drug Development
However, the difference between drug design and drug development is discussed step by step.
Definition of Drug Design vs Drug Development
Definition of Drug design
Drug design is an inventive effort of designing a suitable lead molecule to make it more active and safer with less toxicity than the original lead molecule that may properly bind with its specific receptor sites to produce a certain pharmacological effect.
In other words, Drug design is an inventive effort of designing a suitable lead molecule to improve its pharmacodynamic properties (interaction with its target) as well as Pharmacokinetics properties (ability to reach its target & to bind for an acceptable lifetime). Difference between Pharmacokinetics and Pharmacodynamics.
Simply, Drug design means lead modification, lead is a chemical with potential therapeutic benefit(s). Once the lead compound has been invented it can be used as the starting point for drug design.
Drug design is the inventive process of finding new medications based on the knowledge of a biological target. [1]
The binding affinity of a drug molecule to its receptor sites depends on the structure of the drug molecules. So, the structure of the drug molecule must be suitable to permit binding to certain receptor sites to produce a certain pharmacological effect.
Definition of Drug Development
Drug development is the process of establishing and marketing a safe, stable, and effective new pharmaceutical drug product through preclinical, clinical testing, and regulatory approval, from a biologically active lead compound that has been available via the process of drug discovery and drug design.
In other words, Drug development is the physicochemical and pharmaceutical modification of new or existing active compounds to make the compound with increased stability, activity, selectivity, reduced side effects, and easy and efficient administration to the patient. Drug development includes preclinical, clinical testing, and regulatory approval with a new drug application to market the drug.
Simply, Drug development is the process of establishing and marketing a biologically active compound obtained by drug design.
Types of Drug Design and Drug Development
There are two key types of drug design [2]:
- Direct drug design (Structure-based drug design): depends on the information of the 3D (three-dimensional) structure of the biological target achieved through methods such as NMR spectroscopy or x-ray crystallography.
- Indirect drug design (Ligand-based drug design): depends on the information of other molecules that bind to the biological target of interest.
On the other hand, drug development has no classification.
Aims of Drug Design and Drug Development
Drug design aims to improve the pharmacodynamic properties and pharmacokinetic properties of a drug. There are various aims in drug design:
- To achieve optimum selectivity means optimization of the interaction of the drug with its target (particular cells or tissues),
- Designing a drug with a good level of activity for its target,
- To make the drug with minimum side effects,
- Designing a drug that can be synthesized easily and efficiently with minimum cost,
- Making the drug chemically stable,
- To improve the drug’s non-toxic or reduce its toxicity,
On the other hand, the aims of drug development are:
- Establishing and marketing a safe, stable, and effective new pharmaceutical drug product through preclinical, clinical testing and regulatory approval, from a biologically active lead compound that has been available via the process of drug discovery and drug design.
- Ensuring the safety and effectiveness of a new pharmaceutical drug (Medicine).
- Developing and selecting a suitable dosage form for administering.
Drug Design and Drug Development Method
The drug is designed by following one or more methods:
- Variation of substituents
- Extension of structure
- Ring expansion/contraction
- Chain extension/contraction),
- Ring variation,
- Ring fusion,
- Isosteres and Bioisosteres,
- Simplification of the structure,
- Rigidification of the structure.
- Conformational blockers
- Stereoelectronic modification
- Prodrug synthesis
Whereas, the development process is divided into two sections [3]:
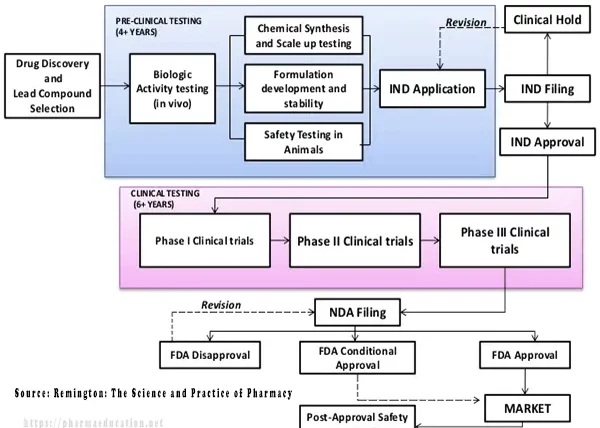
1. Preclinical testing (lead compound selection and animal testing of new chemicals). Preclinical testing includes:
- Discovery testing to ensure biological activity in vivo
- Chemical synthesis and scale-up to ensure adequate quantities of high purity can be made.
- Formulation development and testing to characterize various chemical properties, develop the initial drug delivery system, and determine the stability of the compound.
- Animal safety testing to ensure the limited toxicities of the lead agent.
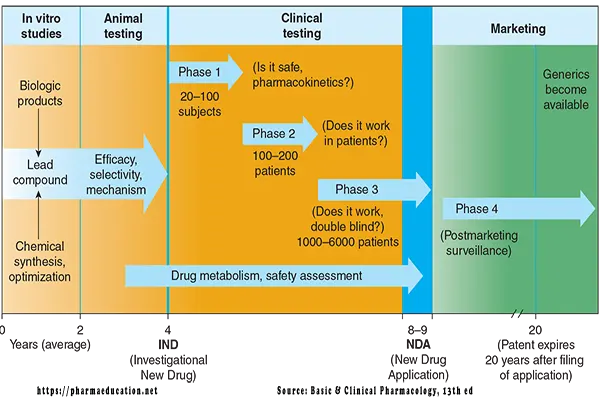

2. Clinical testing (administration of new chemicals to humans).
Cost of drug design and drug development
Drug design is less expensive than drug development. Whereas, the drug development phase is significantly more expensive.
Time required
Drug design may take 0-2 years [4]. Whereas, drug development may take 10+ years (Preclinical testing may take 4+ years and clinical testing may take 6+ years [3] and 1 year for regulatory approval).
Patron
The attempt at Drug design is taken by either governmental research organizations or private research organizations or university research laboratories or pharmaceutical companies. While Attempt to drug development is mostly taken directly or indirectly by pharmaceutical companies (such as Merck & Co., Inc., Pfizer, Roche) once a biologically active compound has been available via the process of drug discovery and drug design.
Example of Drug Design and Drug Development
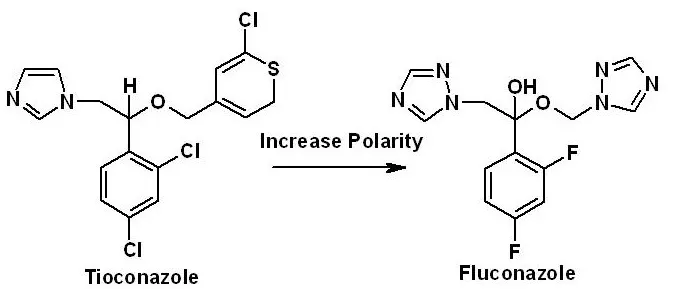
Example of drug design: Tioconazole (antifungal) is a non-polar, imidazole-containing, and poorly absorbed drug. Replacing the imidazole ring (susceptible to metabolism) in Tioconazole with 1, 2, the 4-triazole ring gives the orally active antifungal agent Fluconazole with improved polarity and stability.
Example of drug development: establishing and marketing a safe, stable, and effective new Fluconazole tablet from Fluconazole after preclinical and clinical testing and regulatory authority approval.
In short, the difference between drug design and drug development
| Features | Drug Design | Drug Development |
| Definition | Drug design is an inventive effort of designing a suitable lead molecule to make it more active and safer with less toxicity than the original lead molecule that may properly bind with its specific receptor sites to produce a certain pharmacological effect. | Drug development is the process of establishing and marketing a safe, stable, and effective new pharmaceutical drug through preclinical, clinical testing and regulatory approval, from a biologically active lead compound that has been available via the process of drug discovery and drug design. |
| Types | Drug design is two types. 1. Direct drug design (Structure-based drug design) 2. Indirect drug design (Ligand-based drug design) | No classification |
| Aims | To improve pharmacodynamic properties and pharmacokinetic properties of drug. | Establishing and marketing a safe, stable, and effective new pharmaceutical drug product. |
| Method | Designed by following one or more methods: 1. Variation of substituents 2. Extension of structure 3. Ring expansion/contraction 4. Chain extension/contraction), 5. Ring variation, 6. Ring fusion, 7. Isosteres and Bioisosteres, 8. Simplification of the structure, 9. Rigidification of the structure. 10. Conformational blockers 11. Stereoelectronic modification 12. Prodrug synthesis | To improve pharmacodynamic properties and pharmacokinetic properties of drugs. |
| Cost | Less expensive than drug development | More expensive |
| Time | 0-2 years | 10+ years (For preclinical testing 4+ years and clinical testing 6+ years and 1 year for regulatory approval) |
| Patron | The attempt at Drug design is taken by either governmental research organizations or private research organizations or university research laboratories or pharmaceutical companies. | Attempt to drug development are mostly taken directly or indirectly by pharmaceutical companies (such as Merck & Co., Inc., Pfizer, Roche). |
| Example | Replacing the imidazole ring (susceptible to metabolism) in Tioconazole with 1, 2, the 4-triazole ring gives the orally active antifungal agent Fluconazole with improved stability. | Establishing and marketing a safe, stable, and effective new Fluconazole tablet from Fluconazole after preclinical and clinical testing and regulatory authority approval. |
Also, you may read:
- A. Difference between generic and brand name drugs
- B. Drug Abuse vs Misuse
- C. Difference between Synthesis and Biosynthesis
References
| 1. Madsen U, Krogsgaard-Larsen P, Liljefors T (2002). Textbook of Drug Design and Discovery. Washington, DC: Taylor & Francis. ISBN 978-0-415-28288-8. |
| 2. Reynolds CH, Merz KM, Ringe D, eds. (2010). Drug Design: Structure- and Ligand-Based Approaches (1 ed.). Cambridge, UK: Cambridge University Press. |
| 3. Remington, Joseph P, and Paul Beringer. Remington: The Science and Practice of Pharmacy. 21st edition. Philadelphia: Lippincott Williams & Wilkins, 2005. |
| 4. Katzung, B.G. Basic & Clinical Pharmacology, 13th ed.; McGraw-Hill: New York, NY, USA, 2015. |
| 5. Brunton, Laurence. Goodman & Gilman’s the Pharmacological Basis of Therapeutics, 11th Ed. New York: McGraw-Hill, Health Professions Division, 2005. |
