Friability test of tablets, Granules, Spheroids is a technique used by the pharmaceutical company to test the durability of tablets, Granules as well as Spheroids before coating, packaging, and transportation.
Tablet Friability is the physical condition that describes the tendency of tablets to break into smaller pieces or to detach a percentage of powder or powder loss from the tablet’s outer surface under mechanical and physical stress.
Since some high-hardness tablets tend to produce capping or lamination after compression thus tablet hardness is not an absolute indicator of strength. Therefore, friability is another measure of a tablet’s strength. In the pharmaceuticals industry often friability test is done to determine the friability of compressed, uncoated tablets, but also to determine the friability of Granules and Spheroids. You may also read, List of Pharmacopoeia
Table of Contents
Friability test of tablets (uncoated)
Sample requirement: For ≤ 650 mg weight of tablets, take 6.5 g tablets or as near as possible to 6.5 g. For tablets with more than 650 mg weight, take 10 tablets. Before testing, tablets must be cautiously dedusted.
Friability Test Apparatus [2, 3]
Friability Test Apparatus or Friability tester is known as the Roche friabilator. A Friability Test Apparatus consists of a drum, with an internal diameter between 283 mm – 291 mm and a depth between 36 mm – 40 mm, of transparent synthetic polymer with polished internal surfaces, and subject to minimum static build-up (Figure). Additionally, the drum has a removable side. A curved projection that curves from the center of the drum to the outside wall and has an interior radius between 75.5 mm and 85.5 mm tumbles the tablets with each rotation of the drum.The outer diameter of the central ring is between 24.5 mm – 25.5 mm. The drum is attached to the horizontal axis of a device that rotates at 25 ± 1 rpm. Drums with dual scooping projections, or an apparatus with more than one drum, for the running of multiple samples at one time, are also permitted.
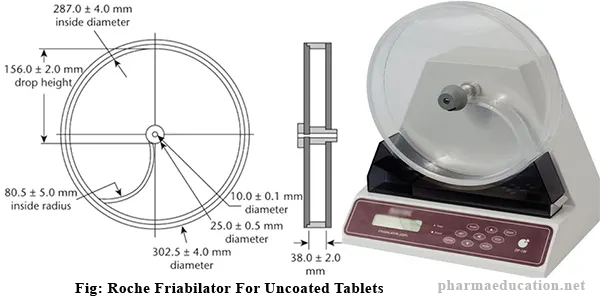
Parts of a Friability Test Apparatus
Friability Test Apparatus has the following different parts:
- Electronic Controller (LED display, DC gear motor, wheel navigator
- Drum/ drums
- Integrated report printer &
- Discharge collector tray
How to perform the Friability Test of tablets? Friability Test Procedure
- Firstly, check the calibration status and clean the Friability Test Apparatus (friabilitor) before starting the operation
- Before testing, tablets must be cautiously dedust.
- For ≤ 650 mg weight of tablets, take 6.5 g tablets or as near as possible to 6.5 g. For tablets with more than 650 mg weight, take 10 tablets. Put the required quantity of tablets (it is initial weight) into the drum.
- Start the test after setting all parameters (revolution time).
- Then the tablets should be dedust after testing and weighed (it is the final weight).
- Finally, apply the following formula to calculate the friability.
The formula for the Friability Test of Tablets
By using the following Friability Test formula, you may calculate the friability of tablets:

Where,
W1 = Initial Weight of Tablets (Before Operation or Tumbling) &
W2 = Final Weight of Tablets /After Operation or Tumbling)
Acceptable Friability Test Limit
According to USP, IP, and BP, a maximum weight loss (obtained from a single test or from the mean of three tests) not more than 1.0% is considered acceptable for most products.
Chewable tablets and Effervescent tablets may have different specifications as far as friability is concerned. In the case of hygroscopic tablets, an appropriate humidity-controlled environment is required for testing.
Generally, the test is run once. If noticeable tablet defects such as cracked, cleaved, or broken tablets are present in the tablet sample after the operation (tumbling), the sample fails the test. If the results are difficult to interpret or if the weight loss is greater than the targeted value, the test should repeat 3 times and calculate the means of it.
Friability Test of Granules and Spheroids [3]
According to BP, two methods are available to determine the friability of granules as well as spheroids under defined conditions:
Method A: Apparatus (Fluidized-Bed Apparatus)
Method B: Apparatus (Oscillating Apparatus)
Friability test formula for Granules and Spheroids
F = m1 (100−T1) − m2 (100 − T2) m1 × 100F = m1 (100 − T1) − m2 (100 − T2) m1 × 100
F = friability;
T1 = percentage loss on drying before the test (mean of 2 determinations);
T2 = percentage loss on drying after the test (mean of 2 determinations);
m1 = mass of the granules or spheroids before the test, in grams;
m2 = mass of the granules or spheroids after the test, in grams.
Name of some brand of Friability Test Apparatus
- ERWEKA Tablet Friability Tester
- Copley Tablet Friability Tester
- PTF Tablet Friability Tester
- HMK-1601 Tablet Friability Tester
- CS-1/2/3 Tablet Friability Tester
- Electrolab Dual Drum Friability Tester
Causes of High friability of tablets
- Inadequate binder.
- Over-drying of granules.
- The use of some excipients such as microcrystalline cellulose, silicified microcrystalline cellulose, magnesium silicate, polysorbate, and sodium stearyl fumarate, gives low friability at lower compression pressures.
- Too much or too little compression pressure.
- Over-lubrication.
- Improper tablet design.
The above factors affect tablet friability. So, this is the answer of: What are the factors affecting the friability of the tablet?
Remedies of High friability of tablets
- Increase binder level or change or stronger binder.
- Dry the granules properly.
- Replace that excipient which is lower friability; make a tablet with optimum hardness.
- Adjust pressure for acceptable friability.
- Use sufficient lubricant with optimum blending time.
- Select the proper tablet shape.
In these ways, you may improve the friability of tablets. Therefore, this is the answer of: how to improve the friability of tablets?
Importance of Friability Test of tablets [1]
- Helps to determine the resistance of compressed tablets during manufacturing (coating), packaging, and shipping.
- It is another way to measure the strength of tablets.
The Friability Test of tablets is the same in United States Pharmacopeia (USP), British Pharmacopoeia (BP), European Pharmacopoeia (Ph. Eur.), India Pharmacopoeia (IP), Japanese Pharmacopoeia (JP) as well as International Pharmacopoeia (Ph. Int.) etc.
Keywords: Friability test of tablets, Friability test of Granules, Spheroids, Friability test.
References
1. Lachman, Lieberman, H.A. and Kanig, J.L., The Theory and Practice of Industrial Pharmacy, Lea and Febiger, Philadelphia, 3rd edn., 1986.
2. United States Pharmacopeia and National Formulary (USP 43–NF 38). Rockville, MD: United States Pharmacopeial Convention; 2020.
3. British Pharmacopoeia Commission. British Pharmacopoeia 2021. London: TSO; 2021.