Cofactor and Coenzyme are essential non-protein molecules for an enzyme. Difference between Cofactor and Coenzyme is described step by step.
Table of Contents
Definition of Cofactor and Coenzyme
Cofactors are either one or more inorganic (e.g. metal ions, iron-sulfur clusters) or complex organic or metalloorganic (e.g. flavin and heme), non-protein chemical compounds that assist in the biochemical transformation of an Apoenzyme.
On the other hand, Coenzymes are small, organic or metalloorganic, non-protein molecules that are as auxiliary for the specific action of an enzyme.
Classification of Cofactor and Coenzyme
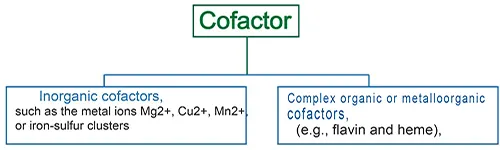
Cofactors are divided into two broad groups:
- Organic cofactors, such as flavin or heme,
- Inorganic cofactors, such as metal ions (Mg2+, Cu2+, Mn2+), or iron-sulfur clusters.
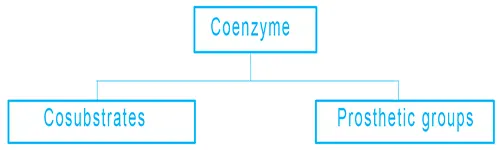
Coenzymes are divided into two categories:
- Cosubstrates: They are temporarily bound to the protein and will be released at some point, then get back in.
- Prosthetic groups: They are bound permanently to the protein.
Chemical nature of Cofactor and Coenzyme
Cofactors are non-protein, metallic ions. They may be either inorganic ions or organic molecules. Additionally, some sources also limit the use of the term “cofactor” to inorganic substances.
On the other hand, Coenzymes are complex organic or metalloorganic, non-protein chemical compounds.
Function of Cofactor and Coenzyme
Cofactors also are known as “helper molecules” that assist apoenzyme during the catalysis of reactions.
While Coenzymes act as a transient carrier of specific functional groups from enzyme to enzyme. Coenzymes bind to the apoenzyme and assist in enzyme activity.
Example of Cofactor and Coenzyme
Cofactors: metal ions Mg2+, Cu2+, Mn2+, or iron-sulfur clusters.
Coenzyme: Biotin, Coenzyme A, NADH, NADPH and adenosine triphosphate (ATP), Riboflavin, Thiamine, and Folic Acid etc.
You may read:
- Enzyme, Coenzyme, Apoenzyme, Holoenzyme, and Cofactor
- Agonist, Partial Agonist, Antagonist, and Inverse Agonist
- Difference between Pharmacokinetics and Pharmacodynamics
Summary of the difference between Cofactor and Coenzyme
| Features | Coenzyme | Cofactor |
|---|---|---|
| Definition | Co-enzymes are small, organic or metalloorganic, non-protein molecules that are as auxiliary for the specific action of an enzyme. | Co-factors are either one or more inorganic (e.g. metal ions and iron-sulfur clusters) or a complex organic or metalloorganic (e.g. flavin and heme), non-protein chemical compounds that assist in the biochemical transformation of an Apoenzyme. |
| Classification | Co-enzymes are divided into two categories: 1. Co-substrates: They are temporarily bound to the protein and will be released at some point, then get back in. 2. Prosthetic groups: They are bound permanently to the protein. | Co-factors are divided into two broad groups: 1. Organic cofactors 2. Inorganic cofactors Organic co-factors are sometimes further divided into co-enzymes and prosthetic groups. |
| Chemical nature | Co-enzymes are complex organic or metalloorganic ,non-protein chemical compounds. | Co-factors are non-protein, metallic ions. They may be either inorganic ions or organic molecules. Additionally, some sources also limit the use of the term “co-factor” to inorganic substances. |
| Function | They act as a transient carrier of specific functional groups from enzyme to enzyme. Co-enzymes bind to the apoenzyme and assist in enzyme activity. | Co-factors also are known as “helper molecules” that assist apoenzyme during the catalysis of reactions. |
| Another name | Transient carrier molecule | Helper molecule |
| Example | Biotin, Co-enzyme A, NADH, NADPH and adenosine triphosphate (ATP), Riboflavin, Thiamine, and Folic Acid. | Metal ions such as Mg2+, Cu2+, Mn2+, or iron-sulfur clusters. |