Tablet defects or manufacturing defects of tablets are not acceptable. But often tablet defects embarrass tablet manufacturers or formulators to design a new tablet. This article outlines various remedies that resolve various tablet defects. We explain various tablet defects with pictures. If you are an industrial pharmacist or work in product development or formulation research and development, you must know about these tablet defects and remedies. A formulator or product developer usually encounters all of the tablet defects during the manufacturing of tablets. Tablet coating defects and remedies.
Presently the tablet is the most broadly used dosage form for drug delivery because of some benefits including economical, patient convenience, and compliance. Tablet defects are either related to functional defects or visual defects or both. Tablet defects are associated with imperfections in any one or more of the following factors:
- Formulation of Tablet
- Tableting equipment
- Manufacturing process of tablets
Table of Contents
List of tablet defects
- Capping
- Lamination
- Chipping
- Sticking
- Picking
- Cracking
- Binding / Binding in the die
- Edging / Flashing of tablet
- Mottling
- High friability
- Weight Variation
- Hardness Variation
- Aging of tablets/ Loss of hardness
- Protracted Hardness (Hardness increases with time)
- Disintegration time too long
- Uneven Breakage
- Black Spot/Stain
- Double Impression
- Bisected/ Score line/ breakline or imprint or logo not sharp or well-defined
- Splitting of Layered tablets or layer separation
- Layers not sharply defined bilayer tablet
Generally, tablet defects are appeared by either one or more causes such as capping is occurred when the granules are too dry and/or the lubricant amount is high. Know all of the tablet defects and remedies and make sure the smooth production of tablets. One by one, all possible tablet defects with pictures and remedies are discussed below:
Capping
Capping is a term used to describe the partial or complete separation of the top or bottom crowns of a tablet from the main body of the tablet [1]. In other words, capping is a laminar splitting along the edge of the crown or band of a compressed tablet [2]. Generally, capping is a common tablet defect during tablet manufacturing.

Causes and Remedies for capping of tablets associated with the formulation
| Causes | Remedies |
| High quantity of fine powder materials in the granules. | Use granular or less fine material such as MCC type 102 or 200 instead of MCC type-101. Make proper granules with an optimum amount of fine powder. |
| Air-entrapment in the granular materials. | Increase the dwell time by reducing the turret speed. |
| Too dry granules. | Dry the granules carefully to obtain optimum LOD. Moisten the granules by the solvent used in granulation. Add hygroscopic excipients to maintain an optimum moisture level. |
| Excessive elastic or waxy excipients in the formulation. | Reduce the amount or change the elastic or waxy excipients. |
| Inadequate quantity of binder or inappropriate binder. | Enhance the quantity of binder. Add dry binder such as pregelatinized starch (starch Rx-1500). |
| Presence of too much lubricant. | Use an optimum amount of lubricant. |
| Too soft granules. | Make granules properly and keep optimum LOD. |
| Granular mass is too cold to compress firmly. | Compressed at room temperature. |
Causes and remedies of capping associated with equipment (punches, dies, & compression machine)
| Causes | Remedies |
| Poor tooling choices such as the use of deep concave or extra deep concave punches. | Use shallow or standard concave punches. |
| Excessive compaction force. | Use optimum compaction force. |
| Incorrect setup of dies and punch such as lower punch remains below the face of the die during ejection. | Set up of dies and punches properly. |
| Improper sweep-off setting. | To facilitate proper ejection, adjust the sweep-off blade suitably. |
Lamination
Lamination is the separation of a tablet into two or more distinct horizontal layers. It is a tablet defect in which a tablet splits or separates into layers.

Causes and Remedies of Lamination of tablet associated with the formulation
| Causes | Remedies |
| High quantity of fine powder materials in the granules. | Use granular or less fine material such as MCC type 102 or 200 instead of MCC type-101. Make proper granules with an optimum amount of fine powder. |
| Too dry granules. | Dry the granules carefully to obtain optimum LOD. Add hygroscopic excipients to maintain an optimum moisture level. |
| Oily, elastic, or waxy excipients in granules. | Mixing properly or change of oily, elastic, or waxy excipients. |
| Air-entrapment in the granular material. | Increase the dwell time by reducing the turret speed. |
| Presence of too much lubricant. | Use an optimum amount of lubricant. |
| Inadequate quantity of binder or inappropriate binder. | Use granular or less fine material such as MCC type 102 or 200 instead of MCC type 101. Make proper granules with an optimum amount of fine powder. |
Causes and remedies of Lamination associated with equipment
| Causes | Remedies |
| Die wall binding | Use an adequate amount of lubricant or use tapered dies. |
| Damaged tooling such as poorly finished dies. | Use finely polished dies. |
| Excessive compaction force. | Use optimum compaction force. |
| Rapid decompression | Use precompression and reduce the final compression pressure. |
Chipping
Chipping is the breaking of tablet edges during the pressing process or during the subsequent handling and coating.

Causes and remedies of chipping related to the formulation
| Causes | Remedies |
| Too dry granules. | Moisten the granules; Add hygroscopic substances. |
| Too much or too low binding. | Optimize binding, or use dry binders. |
| Sticking on punch faces | Dry the granules properly or increase lubrication. |
Causes and remedies of chipping related to equipment
| Causes | Remedies |
| Sharp-edge, elongated tablets | Change the tablet punch |
| Lower punch setting too low at tablet take-off | Adjust the lower punch flush with the die face |
| Poor finish or worn punches and dies | Polish, reface, or replace punches and dies |
| Groove of die worn at compression point. | Polish to open-end, reverse or replace the die. |
| Barreled die (center of the die wider than ends) | Polish the die to make it cylindrical |
| Edge of the punch face turned inside/inward. | Polish the punch edges |
| Tablet sweep-off blade on the feed frameset is too high | Adjust setting |
Sticking
Sticking is a defect of the tablet where the tablet surface sticks to the punch face or adhesion of tablet material to the die wall during compression. Simply, sticking is the adherence of material to the faces of tablet press punches or dies after compression [2]. In sticking, the tablet surface sticks to the lower punch face but a small portion of the tablet surface is not detached or the pitted surface will not occur.

Causes and remedies of sticking related to the formulation
| Causes | Remedies |
| Excess moisture in granules | Properly dry the granules to keep optimum moisture. |
| Inadequate lubrication. | Increase the amount of lubricant, Use microfine lubricants. |
| Too much binder. | Lessen the amount of binder. |
| Too much hygroscopic excipients. | Lessen the humidity in the compression room. |
| Sticky API or sticky excipient. | Make granules by wet granulation properly. Add colloid silicon dioxide or microcrystalline cellulose or change the sticky excipient. |
| Low melting point materials. | Mix low melting point materials by microcrystalline cellulose (MCC). Exchange with higher melting point materials. |
Causes and remedies of sticking related to equipment
| Causes | Remedies |
| Punch concavity too deep. | Decrease punch concavity or use shallow concave or flat-face punches. |
| Embossed letters or logo on the punch surface. | Avoid embossed (letters or logo) punches or Use shallow embossing punches. |
| Punch tips burred. | Refinish or replace the punch. |
| Poor finish on punch faces | Polish punch faces; refinish |
Picking
Picking is the term used when a small amount of material from a tablet is sticking to and being removed from the tablet surface by a punch [1]. In other words, the removal of material from the surface of the tablet, and its adherence to the face of the punch is called picking.

Causes and remedies of picking related to the formulation
| Causes | Remedies |
| Inappropriate dried granules. | Properly dry the granules to keep optimum moisture. |
| Inadequate lubrication. | Increase the amount of lubricant. Use microfine lubricants. |
| Low melting point materials in high concentration. | Mix low melting point materials by microcrystalline cellulose. Exchange with higher melting point materials. |
| Too warm granules when compressing. | Sufficiently cool before compression. compression vs compaction |
| Too much binder. | Lessen the amount of binder. |
| Hygroscopic excipients. | Lessen the humidity in the compression room. |
Causes and remedies of picking related to equipment
| Causes | Remedies |
| Rough or scratched punch surfaces. | Polish faces to a high luster. |
| Engraving or embossing letters or logos on punch faces. | Avoid embossed (letters or logo) punches or Use shallow embossing punches. |
| Bevels or break lines too deep. | Reduce depths and sharpness. |
Cracking
Cracking is a defect of tablets where small, fine cracks are observed on the upper and lower central surface of tablets, or very rarely on the sidewall. Easily visible in the tablet that contains pigment or dye.

Causes and remedies of cracking related to the formulation
| Causes | Remedies |
| Large size of granules. | Reduce granule size. Use proper granular size with sufficient fine materials but fine materials should not be more than 15%. |
| Too dry granules. | Dry properly. Moisten the granules properly. |
| Inadequate binder | Use a proper quantity of binder. |
| Rapid expansion of tablets | Improve granulation. Add dry binders. |
| Elastic excipients in granules. | Exchange or lessen elastic excipients. |
| Granulation too cold. | Compress at room temperature. |
Causes and remedies of cracking related to equipment
| Causes | Remedies |
| Tablet expands on ejection due to air entrapment. | Use tapered die. |
| Deep concavities cause cracking while removing tablets | Decrease punch concavity or use shallow concave or flat-face punches. |
Binding / Binding in the die
Binding is a tablet defect where a tablet adheres, seizes, or tears in the die, or a film is formed on the die area and hindered the ejection of the tablet or splits during the molding/pressing process. The powder adheres to punch edges and dies punches may bind in dies difficult ejection.
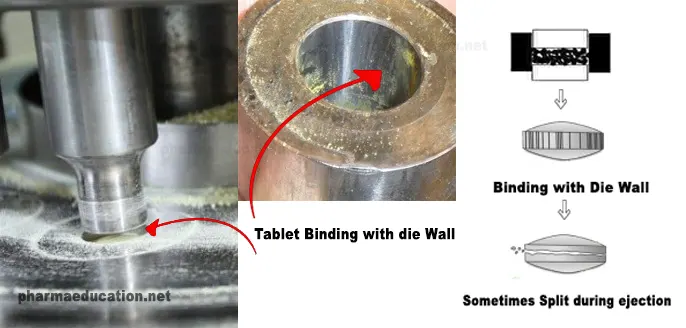
Causes and remedies of binding related to the formulation
| Causes | Remedies |
| Too moist granules. | Dry the granules properly. |
| Excessive binder. | Use proper amount of binder. |
| Inadequate lubrication. | Increase or change lubricant; Increase lubricant blending time. Use microfine lubricants. |
| Too hard granules for the lubricant to be effective. | Modify granulation. Reduce granular size. |
| Granular material very abrasive and cutting into dies. | If coarse granules, reduce their size. Use wear-resistant dies. |
| Use of hygroscopic Ingredients | Process under low humidity conditions |
| Use of Hygroscopic Ingredients | Increase the lubricant level. Add 0.5% Cab-O-Sil® or Syloid |
Causes and remedies of binding related to equipment
| Causes | Remedies |
| Poor finish or worn punches and dies | Polish, reface, or replace tooling |
| Undersized dies. Too little clearance. | Rework to the proper size. Increase clearance. |
Edging / Flashing of tablet
Edging is a defect of tablets in which burrs (raised edges)/ sharp edges appear on the final compressed tablet. This type of tablet defect also known as collaring of the tablet or flashing.

Causes and remedies of Edging / Collaring of tablet / Flashing related to the formulation
| Causes | Remedies |
| Waxy or elastic API or excipients in formulation. | Milled these materials and mix properly. |
| Moisture in granules. | Dry properly. |
Causes and remedies of Edging / Collaring of tablet / Flashing related to equipment
| Causes | Remedies |
| Higher compression pressure | Lessen the compression pressure |
| Poor design punch or wear punch. | Polish, reface, or replace tooling |
Mottling
Mottling is the term used to describe an unequal distribution of color on a tablet, with light or dark spots standing out on an otherwise uniform surface [1]. This type of tablet defect occurs in tablet formulation with a dry coloring agent. Simply, mottling is a tablet defect where dark and light patches on the tablet surface occur.
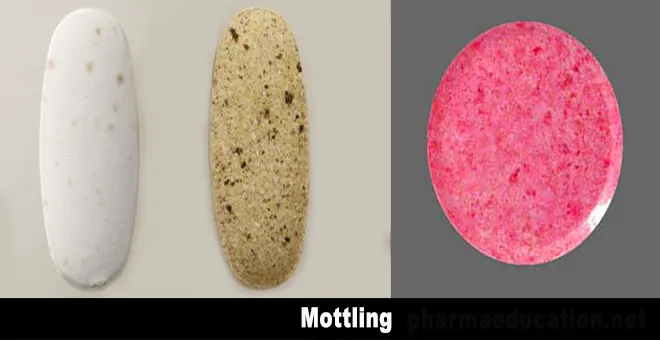
Causes and remedies for mottling
| Causes | Remedies |
| The unequal particle size of the coloring agent or pigment. | Sieved before use to make equal size particles. |
| A colored drug used along with colorless or white-colored excipients. | Use appropriate colorants. |
| A dye migrates to the surface of granulation while drying. | Increase dry mixing time during granulation. Use a proper and fine binder. Use a smaller particle-sized dye. |
| Improperly mixed dye, especially during ‘Direct Compression’. | Mix properly and reduce the size if it is of a larger size to prevent segregation. Use microfine lake dye. Increase blending time. Mill lake dye with 5% excipient. |
| Too small amount of colorant. | Increase the quantity of colorant. |
| Preferential absorption of soluble dye by component of mix. | Replace soluble dye with microfine lake pigment. |
High friability
Causes and remedies of High friability
| Causes | Remedies |
| Inadequate binder. | Increase binder level or change or stronger binder. |
| Over-drying of granules | Dry the granules properly. |
| Some excipients such as microcrystalline cellulose give low friability at lower hardness/machine pressures. | Replace the excipient; make a tablet with optimum hardness. |
| Too much or too little compression pressure. | Adjust pressure for acceptable friability. |
| Over-lubrication | Use sufficient lubricant with optimum blending time. |
Weight Variation
Weight variation is a common defect of tablets that occurs during tablet manufacturing where the average or individual weight of the tablet may be outside of the accepted limit.

According to USP, BP & IP the accepted limit of weight variation given below:
| IP/BP | Accepted Limit | USP |
| Tablet weight 80 mg or less | ± 10% | Tablet weight 130 mg or less |
| More than 80 mg or Less than 250mg | ± 7.5% | 130 mg to 324 mg |
| 250 mg or more | ± 5% | More than 324 mg |
Causes and remedies of Weight Variation
| Causes | Remedies |
| Poor or erratic flow of granules from the hopper. | Make uniform-size granules; Avoid excess fines excipient for direct compression |
| Improper amount of glidant | Use a sufficient amount of glidant. |
| Particle size ranges too wide. | Make uniform-size granules; Avoid excess fines excipient for direct compression |
| Improper drying. | Dry the granules properly. |
| Abnormal uniform mixing of all excipients. | Confirm proper mixing; Increase mixing time. |
| Particle segregation as press RPMs increase | Narrow the particle size range; Make granules by wet granulation. |
| Particle size not suitable for die diameter | Adjust particle size range to recommended optimum for die diameter |
| Lower punch “hang up” (material between the lower punch and die wall or lower punch and punch guide) | Check for proper clearance between the die wall and lower punch; Increase lubricant concentration in the formulation; Remove below 200 mesh fines. |
| Improper tool setting of the machine. Hi-speed running of the machine. | Use optimum speed. |
Hardness Variation
Tablet hardness does not remain constant but it has a range. Hardness variation is a common defect of tablets where individual hardness of tablets may be outside the accepted limit. Unlike weight variation, the hardness variation limit is determined by a prototype trial of that tablet.
Causes and remedies of Hardness Variation
| Causes | Remedies |
| Over-blending. | Optimize the blending time. |
| Weight variation in granules filled in die. | Solve the causes of Weight variation. |
| Improper mixing of lubrication | Mixing properly. |
| Uneven die-fill. | Set the compression machine properly. |
Aging of tablets/ Loss of hardness
It is a tablet defect where the hardness of the tablet decreases with time.
Causes and remedies of Aging of tablets/ Loss of hardness
| Causes | Remedies |
| Over-lubrication | Use sufficient lubricant with optimum blending time; Replace metallic stearates with other lubricants (e.g., stearic acid). |
| Tablets with microcrystalline cellulose (MCC) lose some hardness with time at high humidity, but most of the hardness is quickly regained at normal humidity. | Use moisture scavenger or moisture adsorbent such as calcium silicate; Storage the tablet with normal humidity or replace MCC from tablet formulation. |
| Compression force (pressure) too low | Increase pressure |
| Granulation too soft | Use sufficient binder. |
| Some excipients such as too much starch can give a soft tablet. | Reduce the level of causative excipient. |
| Moisture content too high | Dry the granules properly; Use a moisture scavenger or moisture adsorbent such as calcium silicate. |
| Moisture pick-up | Compress and package under low humidity conditions; Use a moisture scavenger or moisture adsorbent such as calcium silicate. Keep tablet containers well closed. |
Protracted Hardness (Hardness increases with time)
| Causes | Remedies |
| Can be caused by water of crystallization/hydration interacting with ingredients or interactions between active materials-excipients or excipient-excipient. | Conduct a drug-excipients or excipients-excipients compatibility study and replace causing material. |
Disintegration time too long
| Causes | Remedies |
| Over-lubrication. | Use sufficient lubricant with optimum blending time; Replace metallic stearates with other lubricants (e.g., stearic acid). |
| Insufficient disintegrant. | Use super disintegrant; Increase quantity. |
| Too much binder. | Use optimum quantity binder. |
| High hardness tablet. | Reduce machine pressure. |
Uneven Breakage
Many tablets are designed to be broken into smaller dosages with functional score-line / break lines. It is a tablet defect where the tablet breaks unevenly into two parts.

| Causes | Remedies |
| Coarse granules. | Reduce particle size of granulation to less than 16 mesh. |
| Improper mixing. | Increase mixing time. |
| Air-entrapment in the granular materials. | Increase the dwell time by reducing the turret speed. |
| Unequal sizes of granules. | Make granules with sufficient bonder; Milled properly. |
Black Spot/Stain
It is a defect of the tablet where stains or black spots will appear on the tablet’s surface. Mainly observed in colored tablets.
| Causes | Remedies |
| High temperature is the key factor for the penetration of the dye into the upper surface. | Storage tablet at room temperature. |
| Observe in the high concentration of pigment/dyes. | Use an optimum quantity of pigment/dyes. |
| Incompatibility among excipients and API | Replace caustic excipient. |
| Improper cleaning of punches. | Must clean the punches. |
Double Impression
Double impression is machine related tablet defect where a monogram or engraving shape or break line/ score line or imprint or logo appears twice on the tablet surface.

Causes and remedies of double impression
| Causes | Remedies |
| Uncontrolled rotation of either upper punch or lower punch during ejection of a tablet. | Use keying in tooling, i.e. inset a key alongside the punch, so that it fits the punch and prevents punch rotation. |
| Uncontrolled movement of punches with engravings on them. | Using anti-turning devices. |
Bisected/ Score line/ Breakline or Imprint or Logo not sharp or well-defined
| Causes | Remedies |
| Granulation too coarse. | Reduce particle size of granulation. |
| Insufficient binder or binder not strong enough. | Use an adequate quantity of binder or use a stronger binder. |
| Faulty punch debossing design | Redesign using tapered sides on the punch debossing. |
Splitting of Layered tablets or Layer Separation
This type of tablet defect mainly appears in bilayer tablets where the attached layer is separated.
| Causes | Remedies |
| Poor bonding between layers. | Use a stronger binder or sufficient quantity. |
| Compression pressure too high. | Compress at lower pressures. |
| Over-lubrication. | Use sufficient lubricant with optimum blending time. |
| Overdried granules. | Dry granules properly. |
Layers not sharply defined in a bilayer tablet
This type of tablet defect mainly appears in bilayer tablets where the attached layer is not sharply defined.

| Causes | Remedies |
| Coarse granules | Reduce particle size of granulation less than 16 mesh. |
| Too many fines | Remove fines below 200 mesh |
| Insufficient colorant | Use sufficient colorant |
| Improper mixing | Increase mixing time |
So, after reading this article you may encounter all of the tablet defects during the manufacturing of tablets.
Keywords: Tablet defects, Manufacturing defects of the tablet, Tablet defects with pictures, Defects in Tablets, Core Tablet defects, Tablet defects during manufacturing.
References
| 1. Lachman L. Lieberman H. A. & Kanig J. L. (1976). The theory and practice of industrial pharmacy (2d ed.). Lea & Febiger. |
| 2. United States Pharmacopeia and National Formulary (USP 43–NF 38). 2020. Rockville, MD: United States Pharmacopeial Convention. |
Thank you so much. Very useful
What a great great post regarding pharmaceutical tablet defects! I was read many articles about this but this is the most resourceful post. You should use a image of layer separation defect. Thank you.