Pharmacy Abbreviations List: Decoding the Language of Medications. Effective communication is essential in the pharmacy sector for efficient tasking, recordkeeping, as well as patient safety. To facilitate efficient communication among healthcare professionals, ease your tasks like documentation, and convey information quickly and accurately, you must be familiar with pharmacy abbreviations and pharmacy acronyms or pharmaceutical short forms. Also, read the Drug Information website list.
Pharmacy abbreviations act as shorthand language, allowing for the speedy and accurate conveyance of medication-related information in your workplace. However, understanding these acronyms can be difficult, particularly for patients and anyone outside of the healthcare sector. In this article, we present an extensive list of pharmacy abbreviations to assist you in interpreting the language for medications if you work in a pharmacy-related field such as a pharmacist, or pharmacy technician, or work in the pharmaceutical industry or academia. The following are the most well-known pharmacy abbreviations:
Table of Contents
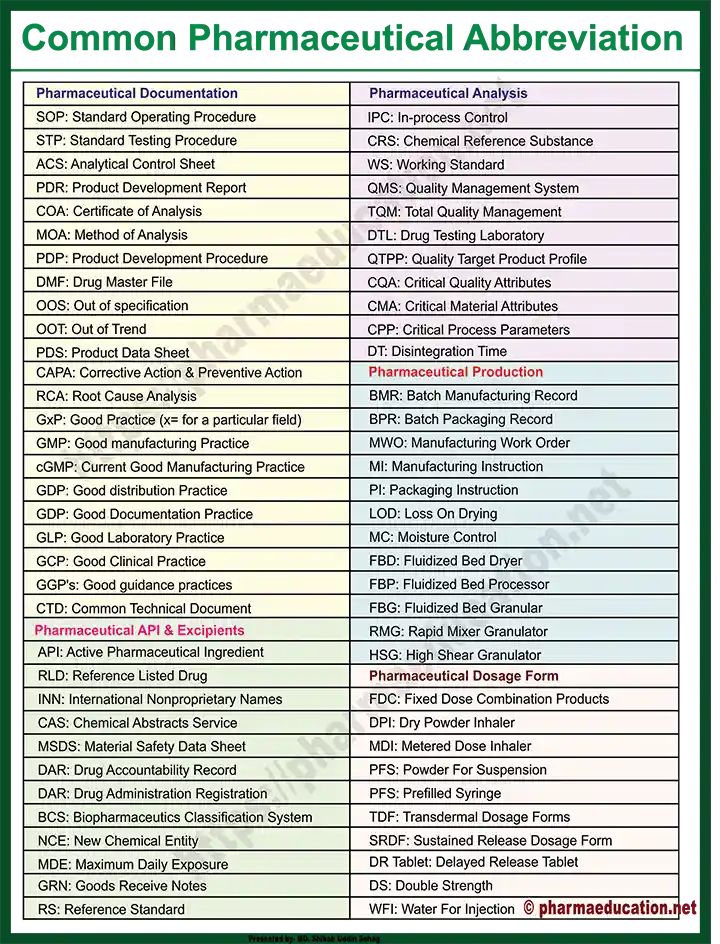
Abbreviations Related to Pharmaceutical Documentation
- SOP: Standard Operating Procedure
- STP: Standard Testing Procedure
- ACS: Analytical Control Sheet
- PDR: Product Development Report
- COA: Certificate of Analysis
- MOA: Method of Analysis
- PDP: Product Development Procedure
- DMF: Drug Master File
- OOS: Out of specification
- OOT: Out of Trend
- PDS: Product Data Sheet
- CAPA: Corrective Action & Preventive Action
- RCA: Root Cause Analysis
- GxP: Good Practice (x= for a particular field)
- GMP: Good Manufacturing Practice
- cGMP: Current Good Manufacturing Practice
- GDP: Good Distribution Practice
- GDP: Good Documentation Practice
- GLP: Good Laboratory Practice
- GCP: Good Clinical Practice
- GGP’s: Good Guidance practices
- CTD: Common Technical Document
Acronym & Pharmacy Abbreviations Related to API & Excipients
- API: Active Pharmaceutical Ingredient
- RLD: Reference Listed Drug
- INN: International Nonproprietary Names
- CAS: Chemical Abstracts Service
- MSDS: Material Safety Data Sheet
- DAR: Drug Accountability Record
- DAR: Drug Administration Registration
- BCS: Biopharmaceutics Classification System
- NCE: New Chemical Entity
- MDE: Maximum Daily Exposure
- GRN: Goods Receive Notes
- NSAID: Nonsteroidal Anti-Inflammatory Drug
- ACE inhibitor: Angiotensin-converting enzyme Inhibitor
- SSRI: Selective Serotonin Reuptake Inhibitor
- ADI: Acceptable Daily Intake
Abbreviations Related to Pharmaceutical Analysis
- IPC: In-process Control
- IPQC: In-process Quality Control
- CRS: Chemical Reference Substance
- RS: Reference Standard
- WS: Working Standard
- PAI: Pharmaceutical Analytical Impurities
- QMS: Quality Management System
- TQM: Total Quality Management
- DTL: Drug Testing Laboratory
- QTPP: Quality Target Product Profile
- CQA: Critical Quality Attributes
- CMA: Critical Material Attributes
- CPP: Critical Process Parameters
- DT: Disintegration Time
Pharmaceutical Production
- BMR: Batch Manufacturing Record
- BPR: Batch Packaging Record
- MWO: Manufacturing Work Order
- MI: Manufacturing Instruction
- PI: Packaging Instruction
- LOD: Loss On Drying
- MC: Moisture Control
- FBD: Fluidized Bed Dryer
- FBP: Fluidized Bed Processor
- FBG: Fluidized Bed Granular
- RMG: Rapid Mixer Granulator
- HSG: High Shear Granulator
List of Pharmacy Abbreviations Related to Dosage Form
- FDC: Fixed Dose Combination Products
- DPI: Dry Powder Inhaler
- MDI: Metered Dose Inhaler
- PFS: Powder For Suspension
- PFS: Prefilled Syringe
- TDF: Transdermal Dosage Forms
- LVP: Large Volume Parenteral
- SVP: Small Volume Parenteral
- SRDF: Sustained Release Dosage Form
- DR Tablet: Delayed Release Tablet
- MR Tablet: Modified Release Tablet
- DS: Double Strength
- WFI: Water For Injection
Pharmacy Abbreviations List Related to Route of Administration
- PO: (Per Os) by the mouth
- IA: Intraarterial
- IP: Intraperitoneal
- IV: Intravenous
- IVP: Intravenous push
- SL: Sublingual
- SubQ or SC: Subcutaneous
- IM: Intramuscular
- ID: Intradermal
- TD: Transdermal
- INH: Inhaled
- NG: Nasogastric
- IT: Intrathecal
- AU: Auricular (Otic)
- EPI: Epidural
- IU: Intrauterine
- OU: Ophthalmic
- PV: Vaginal
- PR: Rectum
- TOP: Topical
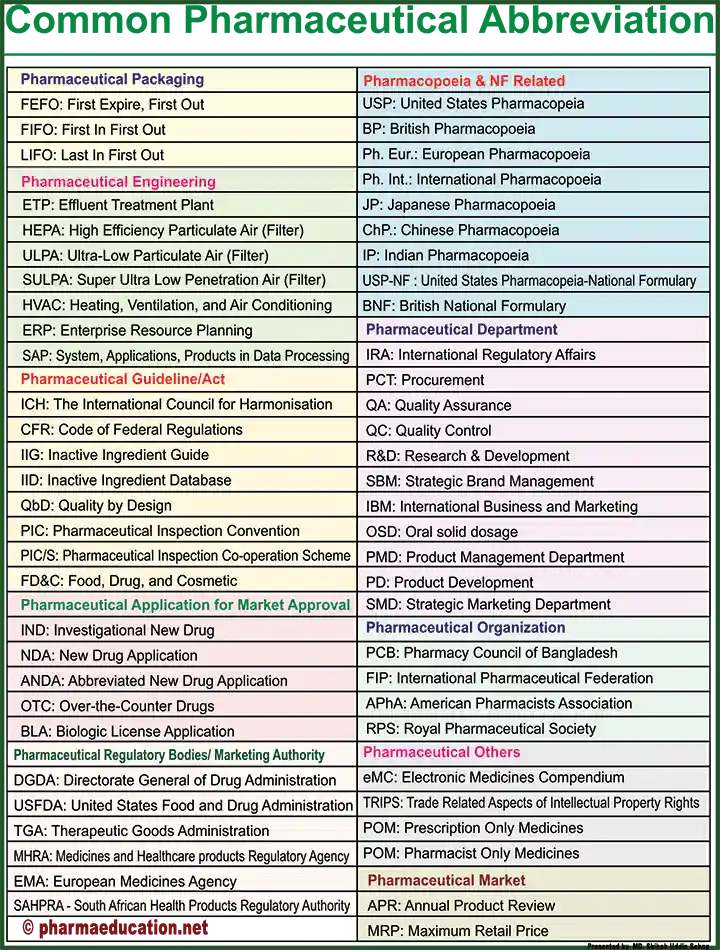
Short Form Related to Packaging
- FEFO: First Expire, First Out
- FIFO: First In First Out
- LIFO: Last In First Out
- POM: Prescription Only Medicines
- POM: Pharmacist Only Medicines
- Rx: ‘Recipere’ meaning “to take”
Pharmaceutical Engineering
- ETP: Effluent Treatment Plant
- HEPA: High-Efficiency Particulate Air (Filter)
- ULPA: Ultra-Low Particulate Air (Filter)
- SULPA: Super Ultra Low Penetration Air (Filter)
- HVAC: Heating, Ventilation, and Air Conditioning
- ERP: Enterprise Resource Planning
- SAP: System, Applications, Products in Data Processing.
Abbreviations related to Pharmaceutical Guidelines/Act
- ICH: The International Council for Harmonisation
- CFR: Code of Federal Regulations
- IIG: Inactive Ingredient Guide
- IID: Inactive Ingredient Database
- QbD: Quality by Design
- PIC: Pharmaceutical Inspection Convention
- PIC/S: Pharmaceutical Inspection Co-operation Scheme
- FD&C: Food, Drug, and Cosmetic
Acronym of Pharmaceutical Application for Market Approval
- IND: Investigational New Drug
- NDA: New Drug Application
- ANDA: Abbreviated New Drug Application
- OTC: Over-the-Counter Drugs
- BLA: Biologic License Application
Pharmaceutical Regulatory Bodies/ Marketing Authorization
- DGDA: Directorate General of Drug Administration
- USFDA: United States Food and Drug Administration
- TGA: Therapeutic Goods Administration
- MHRA: Medicines and Healthcare Products Regulatory Agency
- EMA: European Medicines Agency
- SAHPRA: South African Health Products Regulatory Authority.
Pharmacy Abbreviations Related to Pharmacopoeia & NF-Related
- USP: United States Pharmacopeia
- BP: British Pharmacopoeia
- Ph. Eur.: European Pharmacopoeia
- Ph. Int.: International Pharmacopoeia
- JP: Japanese Pharmacopoeia
- ChP.: Chinese Pharmacopoeia
- IP: Indian Pharmacopoeia
- USP-NF: United States Pharmacopoeia-National Formulary
- BNF: British National Formulary
Abbreviations list of Pharmaceutical Department
- IRA: International Regulatory Affairs
- PCT: Procurement
- QA: Quality Assurance
- QC: Quality Control
- R&D: Research & Development
- SBM: Strategic Brand Management
- IBM: International Business and Marketing
- OSD: Oral solid dosage
- PMD: Product Management Department
- PD: Product Development
- SMD: Strategic Marketing Department
Pharmaceutical Organization
- PCB: Pharmacy Council of Bangladesh
- FIP: International Pharmaceutical Federation
- APhA: American Pharmacists Association
- RPS: Royal Pharmaceutical Society
Abbreviations of Pharmaceutical Marketing
- APR: Annual Product Review
- MRP: Maximum Retail Price
Pharmaceutical Others
- eMC: Electronic Medicines Compendium
- TRIPS: Trade-Related Aspects of Intellectual Property Rights
In conclusion, whether you’re a healthcare professional, a pharmacy student, a pharmacy technician student, or simply a curious individual looking to broaden your knowledge of pharmacy-related terms, this presentation of the pharmacy abbreviations list will be a great reference tool. So, let’s look into the pharmacy abbreviations list and crack the code behind these shortened expressions, allowing you to traverse the world of pharmaceuticals with confidence and clarity. Patients, caregivers, and anyone involved in healthcare can improve their understanding and play a more active part in medication preparation and adherence by being familiar with the pharmacy abbreviations list.
Keywords: Common Pharmaceutical Abbreviation, Pharmaceutical Abbreviations, Pharmacy Abbreviations, Pharmacy Abbreviations List, Pharmacy Tech Abbreviation, Pharmaceutical Short Form, List of Pharmacy Abbreviations, Pharmacy Acronym.
Written by- Md. Shihab Uddin Sohag, RPh.; M. Pharm; B. Pharm.