Sterile and pyrogen-free are the most common criteria of pharmaceutical liquid preparations, especially for parenteral products. Therefore, a health professional must have a clear knowledge of these two words sterile and pyrogen-free. First of all, you need to understand the following terms:
However, if you read this article carefully, you may understand the difference between sterile and pyrogen-free. To clarify the difference between sterile and pyrogen-free, read below points.
Table of Contents
Definition of Sterile and Pyrogen-free
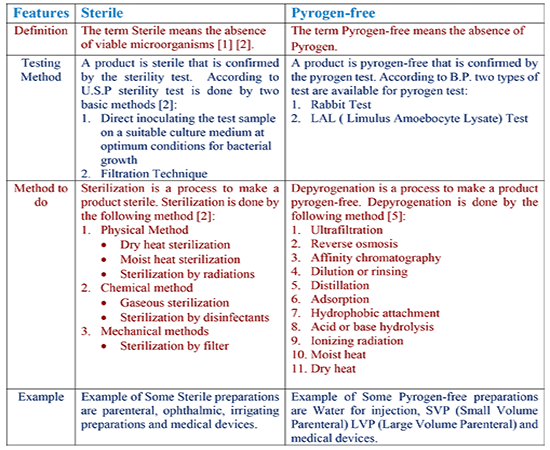
Firstly, the term Sterile means the absence of viable microorganisms [1] [2]. Viable means the ability to proliferate through binary fission under precise conditions. In other words, viable microorganisms mean a cell able to live and multiply. Certainly, sterile does not mean pyrogen-free.
While the term Pyrogen-free means the absence of pyrogen. Pyrogen is a group of substances that cause a rise in temperature in an animal body. Mainly pyrogens are two types: exogenous and endogenous pyrogens [3]. In other words, pyrogen is a fever-inducing agent produced by bacteria, molds, viruses, and yeasts [4]. Further, pyrogens are the metabolic product of microorganisms. In addition, chemically pyrogens are lipid substances associated with a carrier molecule, which is usually polysaccharide but may be peptide [1].
Testing Method
Secondly, sterility test confirms that a product is sterile. In fact, sterility tests are performed on products and materials subjected to a previously validated sterilization procedure. According to the United States Pharmacopeia (U.S.P) sterility test is done by two basic methods [2]:
- Direct inoculating the test sample on a suitable culture medium at optimum conditions for bacterial growth
- Filtration Technique
On the other hand, a product is pyrogen-free that is confirmed by the pyrogen test. According to British Pharmacopoeia (B.P.) two types of testing methods are available for pyrogen test:
- Rabbit Test
- LAL ( Limulus Amoebocyte Lysate) Test
Method to make Sterile and Pyrogen-free
Sterilization is a process to make a product sterile. In other words, it is a process of killing or removing bacteria and other forms of living microorganisms such as fungi, bacteria, and viruses and their spores. It is done by the following method [2]:
1. Physical Method
- Dry heat sterilization
- Moist heat sterilization
- Sterilization by radiations
2. Chemical method
- Gaseous sterilization
- Sterilization by disinfectants
3. Mechanical methods
- Sterilization by filter
While depyrogenation is a process to make a product pyrogen-free. In other words, it is the process to eliminate pyrogens from the pharmaceutical preparations such as water for injection, SVP (Small Volume Parenteral), LVP (Large Volume Parenteral) as well as medical devices. Dehydrogenation is done by the following method [5]:
- Ultrafiltration
- Reverse osmosis
- Affinity chromatography
- Dilution or rinsing
- Distillation
- Adsorption
- Hydrophobic attachment
- Acid or base hydrolysis
- Ionizing radiation
- Moist heat
- Dry heat
Finally, example of Sterile and Pyrogen-free
Sterile products are most frequently solutions or suspensions but may even solid pellets for tissue implantation. Example of some sterile preparations is parenteral, ophthalmic and irrigating preparations. Example of some Pyrogen-free preparations is water for injection, SVP (Small Volume Parenteral) and LVP (Large Volume Parenteral).
However, in the pharmaceutical industry, some preparations may be sterile or sterile and pyrogen-free or pyrogen-free but non-sterile. Visitors are also reading: Drug vs Medicine
Summary of the difference between Sterile and Pyrogen-free
| FEATURES | STERILE | PYROGEN-FREE |
|---|---|---|
| Definition | The term Sterile means the absence of viable microorganisms [1] [2]. | The term Pyrogen-free means the absence of Pyrogen. |
| Testing Method | A product is sterile that is confirmed by the sterility test. According to U.S.P sterility test is done by two basic methods [2]: 1. Direct inoculating the test sample on a suitable culture medium at optimum conditions for bacterial growth 2. Filtration Technique. | A product is pyrogen-free that is confirmed by the pyrogen test. According to B.P. two types of test are available for pyrogen test: 1. Rabbit Test 2. LAL ( Limulus Amoebocyte Lysate) Test |
| Method to do | Sterilization is a process to make a product sterile. Sterilization is done by the following method [2]:
1. Physical Method 2. Chemical method 3. Mechanical methods | Depyrogenation is a process to make a product pyrogen-free. Depyrogenation is done by the following method [5]:
1. Ultrafiltration |
| Example | Example of Some Sterile preparations are parenteral, ophthalmic, irrigating preparations and medical devices | Example of Some Pyrogen-free preparations are Water for injection, SVP (Small Volume Parenteral) LVP (Large Volume Parenteral) and medical devices |
Image of the difference between Sterile and Pyrogen-free
References
- Lechman, Lieberman, Kanig (1976). The theory and practice of industrial pharmacy (2nd Edition). Philadelphia, USA: Lea and Febiger
- Ashok K. G. (1994). Introduction to Pharmaceutics-I (3rd Edition). New Delhi: S.K. Jain
- Anochie, Philip Ifesinachi (2013). Mechanisms of fever in humans. International Journal of Microbiology and Immunology Research Vol.2 (5), pp. 037-043
- (n.d.) Farlex Partner Medical Dictionary. (2012). Retrieved January 22, 2019 from https://medical-dictionary.thefreedictionary.com/pyrogen
- Sandle, Tim. (2011). A Practical Approach to Depyrogenation Studies Using Bacterial Endotoxin. Journal of GXP Compliance. 15. 90-96
data about sterile and pharamceuticals are useful