Etymologically the word probiotic was derived from the Latin preposition pro (“for” or “in support”) and the Greek adjective (biotic) from the noun bios (“life”) meaning ‘for life’ or ‘in support of life’ [1].
In 1907, the concept behind probiotics was introduced by the Russian-born Nobel laureate Elie Metchnikoff, known as the “father of probiotics, who suggested that “The dependence of the intestinal microbes on the food makes it possible to adopt measures to modify the flora in our bodies and to replace the harmful microbes by useful microbes” [2]. After that, in 1954, Vergin first introduced the term “probiotics”, when he compared in his paper “Anti- and Probiotika”, the detrimental effects of antibiotics and other antimicrobial substances on the gut microbial population with factors “probiotika” favorable to the gut microflora [3].
Originally the word probiotic was used to describe substances produced by one protozoan which stimulated another. But chronologically the concept of probiotic is changed and nowadays probiotics are defined as live microorganisms which, when administered in appropriate amounts, may confer health benefits to their host generally by improving or restoring the gut flora. However, the word probiotic has been used in a number of different ways over the years.
Table of Contents
A Chronicle of the Evolution of the definition of Probiotics
| Years | Definitions |
|---|---|
| 1965 | Products that contain live organisms shown in reproducible human studies to confer a health benefit can actually claim to be probiotics [18]. |
| 1971 | Tissue extracts that stimulate microbial growth [5]. |
| 1974 | Organisms and substances that have a beneficial effect on the host animal by contributing to its intestinal microbial balance [6]. |
| 1989 | A live microbial feed supplement that beneficially affects the host animal by improving its intestinal microbial balance [7]. |
| 1992 | A viable mono- or mixed culture of microorganisms that, applied to animals or humans, beneficially affects the host by improving the properties of the indigenous microflora [8]. |
| 1996 | A live microbial culture of cultured dairy product that beneficially influences the health and nutrition of the host [9]. Viable bacteria, in a single or mixed culture, that has a beneficial effect on the health of the host [10]. |
| 1998 | Living microorganisms that on ingestion in certain numbers exert health benefits beyond inherent basic nutrition [11]. |
| 1999 | A microbial dietary adjuvant that beneficially affects the host physiology by modulating mucosal and systemic immunity, as well as improving nutritional and microbial balance in the intestinal tract [12]. |
| 2001 | A preparation of or a product containing viable, defined microorganisms in sufficient numbers that alter the microflora (by implantation or colonization) in a compartment of the host and by that exert beneficial health effects in this host [13]. |
| 2002 | Specific live or inactivated microbial cultures that have documented targets in reducing the risk of human disease or in their nutritional management [14]. Live microorganisms which, when ingested in appropriate amounts, may confer health benefits to their host. [15]. |
| 2002 | Specific live or inactivated microbial cultures that have documented targets in reducing the risk of human disease or in their nutritional management [14]. Live microorganisms which, when ingested in appropriate amounts, may confer health benefits to their host. [15]. |
| 2004 | Preparation of viable microorganisms that is consumed by humans or other animals with the aim of inducing beneficial effects by qualitatively or quantitatively influencing their gut microflora and/or modifying their immune status [16]. |
| 2006 | Probiotics are human-associated microorganisms which are consumed either with food or as a supplement with the purpose of improving the health of the host [17]. |
| 2010 | Products that contain live organisms shown in reproducible human studies to confer a health benefit can actually claim to be probiotic [18]. |
| 2013 | Probiotics are live microorganisms that may exert beneficial effects regarding the sufficiency of consumption via their impact on the microbial balance of the gut [19]. |
| 2018 | Probiotics are live microorganisms that are intended to provide health benefits when consumed, generally by improving or restoring the gut flora. Products sold as probiotics include foods (such as yogurt), dietary supplements, and products that aren’t used orally, such as skin creams [20]. |
Criteria of Good Probiotics
There are a number of criteria that must meet during the selection of a probiotic bacterial strain with the utmost importance placed on safety issues. Strains of the Lactobacillus and Bifidobacterium genera are usually regarded as safe from the basis of long-term human use.
In 1989, Fuller [7] listed the following as criteria of a good probiotic:
- Probiotics should be a strain, which is capable of exerting a beneficial effect on the host, for example, increased growth or resistance to disease.
- It should be non-pathogenic and non-toxic.
- They should be present as viable cells, preferably in large numbers.
- It should be capable of surviving and metabolizing in the gut environment, for example, it should be resistant to low pH and organic acids.
- They should be stable and capable of remaining viable for periods under storage and field conditions.
- A probiotic must be alive when administered [21].
- It must have undergone controlled evaluation to document health benefits in the target host [22].
- Probiotics should adhere to the human intestinal cells.
- It should colonize of the human intestinal tract.
- They should antagonize against pathogenic bacteria.
- It must be safe for the human which means the bacteria may generally recognize as safe (GRAS) and the safety of the potential probiotic should assess by the determination of antibiotic resistance patterns and certain metabolic activities.
How do Probiotics work?
The exact mechanisms by which probiotics accomplish their beneficial actions are unknown. Consumption of probiotics may associate with immune system stimulation, decreased cholesterol blood levels, protection against respiratory and intestinal diseases, reduction of inflammatory responses, and antitumorigenic effects. These alleged health claims apparently stem from the ability of probiotics to secrete antimicrobial substances, compete with other pathogens, strengthen the intestinal barrier and modulate the immune system [23]. Four different mechanisms under research by which probiotics may defend against pathogens in the intestine [24].
Competition for nutrients
Probiotics may compete against pathogens for the same essential nutrients, leaving less available for the pathogen to utilize (Fig 1).
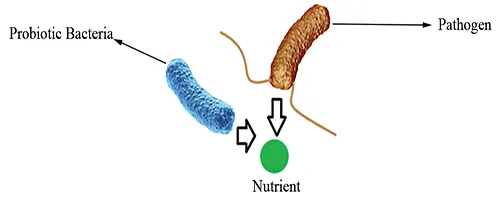
Figure 1: Competition for nutrients
Blocking of Adhesion Sites
They may bind to adhesion sites, preventing pathogen attachment by reducing the surface area available for pathogen colonization (Fig 2).
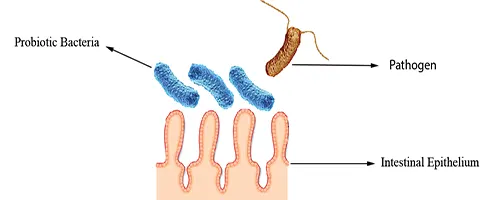
Figure 2: Blocking of Adhesion Sites
Immune Stimulation
Specific strains of probiotics may influence the innate and the acquired immune system, thus playing an important role in human diseases. Probiotic bacteria may affect the epithelial cells, the dendritic cells, the monocytes or macrophages and the several types of lymphocytes (Natural killer cells, T-cells and T-cell redistribution) directly or secondarily. Signaling of immune cells by probiotics may result in the secretion of cytokines, targeting the pathogen for destruction (Fig 3).
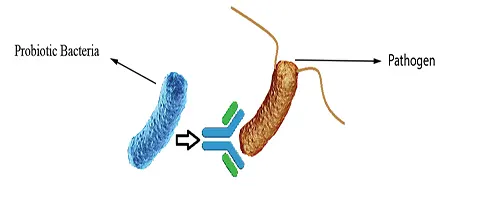
Figure 3: Immune Stimulation
Direct Antagonisms
Probiotics may attack pathogenic organisms by releasing antimicrobial agents such as bacteriocins and killing them directly (Fig 4).
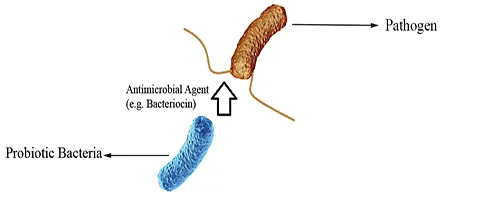
Figure 4: Direct Antagonisms
Visitors are also read: Incredible 16 Health Benefits of Probiotics and their Natural Sources
Examples of common probiotic microorganisms
There are many different microorganisms currently used as probiotics (Table 1) including yeast [25], bacilli [26], Escherichia coli [27, 37], enterococci [28], and the more commonly used bifidobacteria and lactic acid bacteria, such as lactobacilli, lactococci and streptococci [29]. Lactobacillus and Bifidobacterium are two common inhabitants of the human intestinal microbiota [30]. They are known to have no harmful effects, which is in contrast to other gut bacteria [31].
Table 1: Common probiotic microorganisms [32-38]
| Genus | Species |
| Lactobacillus | L. acidophilus, L. delbrueckii ssp. bulgaricus, L. fermentum L. crispatus, , L. gallinarum, L. gasseri, L. johnsonii, L. brevis, L. plantarum, L. reuteri, L. rhamnosus, L. salivarius, L. casei, L. cellobiosus, L. curvatus, L. amylovorus, L. helveticus |
| Enterococcus | Enterococcus faecium, Enterococcus faecalis |
| Lactococcus | Lactococcus lactis |
| Leuconostoc | Le. citreum, Le. mesenteroides subsp. cremoris |
| Pediococcus | Pd. acidilactici, Pd. pentosaceus |
| Sporolactobacillus | Sporolactobacillus inulinus |
| Streptococcus | S. thermophiles, S. faecalis S. faecium, S. salivarius |
| Bifidobacterium | Bf. adolescentis, Bf. animalis, Bf. bifidum, Bf. breve, Bf. Infantis, Bf. longum |
| Bacillus | Bc. cereus, Bc. coagulans, Bc. clausii, Bc. pumilus, Bc. subtilis, |
| Escherichia | Escherichia coli Nissle 1917 |
| Propionibacterium | Pr. acidipropionici, Pr. freudenreichii subsp. shermanii, Pr. jensenii, |
| Saccharomyces | Saccharomyces cerevisiae, Saccharomyces boulardii |
References
1. Hamilton-Miller JM, Gibson GR, Bruck W (2003). Some insights into the derivation and early uses of the word ‘probiotic’. Br. J. Nutr, 90 (4), 845.
2. Metchnikoff E (1907). Lactic acid as inhibiting intestinal putrefaction. In: The prolongation of life: Optimistic studies. W. Heinemann, London: 161-183.
3. Vergin F. (1954). Anti- and probiotika. Hippokrates 25, 16–19.
4. Lilly, D .M. & Stillwell, R. H. (1965) Probiotics: Growth promoting factors produced by microorganisms. Science 147, 747-748.
5. Sperti GS (1971). Probiotics. West Point (CT): AVI Publishing Co. 1971.
6. Parker, R. B. (1974) Probiotics, the other half of the antibiotics story. Animal Nutrition and Health 29, 4-8.
7. Fuller R (1989). Probiotics in man and animals. J. Appl. Bacteriol, 66, 365-378.
8. Havenaar R (1992). Huis In’t Veld JMJ. Probiotics: a general view. In: Wood BJB, ed. Lactic acid bacteria in health and disease. Vol 1. London: Elsevier Applied Science Publishers (151-70).
9. Salminen S (1996). Uniqueness of probiotic strains. International Dairy Feeding Sand Nutrition Newsletter. 5, 16-18.
10. Donohue DC, Salminen S. (1996). Safety of probiotic bacteria. Asia Pac. J. Clin. Nutr. 5, 25–8.
11. Guarner F, Schaafsma GJ (1998). Probiotics. Int. J. Food Microbiol. 39, 237–8.
12. Naidu AS, Bidlack WR, Clemens RA (1999). Probiotic spectra of lactic acid bacteria (LAB). Crit. Rev. Food Sci. Nutr. 39, 13-126
13. Schrezenmeir, J.; De Vrese, M. (2001). Probiotics, prebiotics, and synbiotics: Approaching a definition. American Journal of Clinical Nutrition,73, S361–S364.
14. Isolauri E, Rautava S, Kalliomaki M (2002). Role of pro-biotics in food hypersensitivity. Current Opinion in Immunological Clinical Allergy, 2, 263-271.
15. FAO/WHO. (2002). Guidelines for the evaluation of probiotics in food. Retrieved August 23, 2006, from/ ftp://ftp.fao.org/es/esn/food/wgreport2.pdfS.
16. Fuller R (2004). What is a probiotic?. Biologist, 51, 232
17. Floch MH, Madsen KK, Jenkins DJ (2006). Recommendations for probiotic use. J Clin Gastroenterol, 40, 275–278.
18. Reid G, Gaudier E, Guarner F, Huffnagle GB, Macklaim JM, Munoz AM, Martini M, Ringel-Kulka T, Sartor B, Unal R, Verbeke K, Walter J (2010). Responders and non-responders to probiotic interventions: how can we improve the odds?. Gut Microbes, 1(3), 200–4.
19. Hemarajata P, Versalovic J. (2013). Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol, 6(1),39-51.
20. US National Institutes of Health (2018). Probiotics: In Depth. National Center for Complementary and Integrative Health.
21. Fuller R (1991). Probiotics in human medicine. Gut. 32(4), 439–42.
22. Food and Agriculture Organization of the United Nations (FAO) (2002). Guidelines for the Evaluation of Probiotics in Food. htttp://ftp.fao.org/es/esn/food/wgreport2.pdf
23. Gill H, Prasad J: Probiotics, immunomodulation, and health benefits. Adv Exp Med Bio 2008, 606:423–454.
24. Bermudez-Brito, M; Plaza-Díaz, J; Muñoz-Quezada, S; Gómez-Llorente, C; Gil, A. (2012). Probiotic mechanisms of action. Annals of Nutrition and Metabolism, 61(2), 160–74.
25. Periti P., Tonelli F. (2001). Preclinical and clinical pharmacology of biotherapeutic agents: Saccharomyces boulardii. J. Chemother. 13, 473–493.
26. Pinchuk I.V., Bressolier P., Vernuil B. (2001). In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents Chemother. 45, 3156–3161.
27. Midtved T. (1997). Stellung von Escherichia coli in der Darmökologie: Bestandteil der Mukosabarriere. Symposiumsband zum 3. Interdisc. Symposium Darmfl ora in Symbiose und Pathogenität, Ansbach, 15–20.
28. Lund B., Edlund C. (2001). Probiotic Enterococcus faecium strain is a possible recipient of the vanA gene cluster. Clin. Infect. Dis. 32, 1384–1385.
29. Mombelli, B. and Gismondo, M.R. (2000). The use of probiotics in medical practice. International Journal of Antimicrobial Agents 16, 531-536.
30. Bielecka M., Biedrzycka E., and Majkowska A. (2002). Selection of probiotics and probiotics for synbiotics and confirmation of their in vivo effectiveness. Food Research International 35, 125-131.
31. Kimoto-Nira H., Mizumachi K., Nomura M., Kobayashi M., Fujita Y., Okamoto T., Suzuki I., Tsuji N.M., Kurisaki J., and Ohmomo S. (2007). Review: Lactococcus sp. as potential probiotic lactic acid bacteria. Japan Agricultural Research Quarterly 41, 181-189.
32. Huys G, Botteldoorn N, Delvigne F. (2013). Microbial characterization of probiotics–Advisory report of the Working Group “8651 Probiotics” of the Belgian Superior Health Council (SHC). Molecular Nutrition & Food Research, 57(8), 1479-1504.
33. Holzapfel W.H., Haberer P., Geisen R., Björkroth, Schillinger U. (2001). Taxonomy and important features of probiotic microorganisms in food and nutrition. Am. J. Clin. Nutr, 73, 365–373.
34. Fuller, R. (1997). Probiotics 2—applications and practical aspects. London: Chapman & Hall.
35. Kurmann, J.A. (1988). Starters for fermented milks. Section 5: Starters with selected intestinal bacteria. In: Fermented milks (science and technology), Bulletin of the International Dairy Federation No 227/1988, p. 41-55.
36. Goldin BR. (1998). Health benefits of probiotics. The British Journal of Nutrition, 80(4), S203–7.
37. Macfarlane GT, Cummings JH. (1999). Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? BMJ (Clinical Research ed), 318(7189), 999–1003.
38. Sonnenborn, U. (2016). Escherichia coli strain Nissle 1917-from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties”. FEMS Microbiology Letters, 363(19.
Great article about probiotics, recently I read an article and it says: Deregulation of the gut microbiota results in various pathological disorders such as diabetes, inflammation, cancer, dyslipidemia, etc. Modulation of intestinal microbiota by probiotics may facilitate the management of a number of clinical conditions of diabetes.
Link: Sohag, M.S.U., Paul, M., Al-Bari, M.A.A., Wahed, M.I.I. and Khan, M.R.I. (2019) Potential Antidiabetic Activities of Probiotic Strains, L. acidophilus and L. bulgaricus against Fructose-Fed Hyperglycemic Rats. Food and Nutrition Sciences, 10, 1419-1432. https://doi.org/10.4236/fns.2019.1012101
You may read to enrich your article. Thank you.