Disintegrants are the most vital excipient in pharmaceutical formulations. They are also known as disintegrating agents or disintegrators. The process by which a solid oral dosage form such as a tablet breaks down into small particles is called disintegration. Oral solid dosage forms such as tablets(Immediate-release tablets, Mosidied release tablets), capsules, beads, pellets, and, granules need to break down into small particles to a rapid release of the drug so that the drug is readily available to dissolve in gastrointestinal fluid. According to USP-NF, disintegration time must be 15 minutes for core tablets, and 30 minutes for film-coated tablets, and hard gelatin capsules. Certainly, to maintain the proper disintegration time of the dosage form a formulator will use the optimum quality of disintegrants.
What are disintegrants or disintegrating agents or disintegrators?
Disintegrating agents or disintegrators or disintegrants are the substances that are added to an oral solid dosage form such as tablets, beads, pellets, granules as well as capsules to promote its rapid disintegration or break down into small particles after administration for facilitating rapid dissolution into GI fluid.
Classification of Disintegrants
Generally, disintegrants are classified into two groups:
- Traditional Disintegrants: such as Starch, Microcrystalline Cellulose, and Sodium Alginate etc.
- Super Disintegrants: such as Crospovidone (cross-linked povidone), Croscarmellose Sodium (cross-linked cellulose) and Sodium Starch Glycolate (cross-linked starch) etc. At this time, these 3 super disintegrants are the most widely used disintegrant in pharmaceutical preparations. Most noteworthy, super disintegrants can swell up 10-fold within 30 seconds.
Further, Super disintegrants are two types:
- Natural Super disintegrants.
- Synthetic Super disintegrants
Generally, disintegrating agents are added before or after wet granulation and/or both in many cases. When disintegrating agents come into contact with a fluid/water either they swell up or wick and then break down, thus facilitating dissolution.
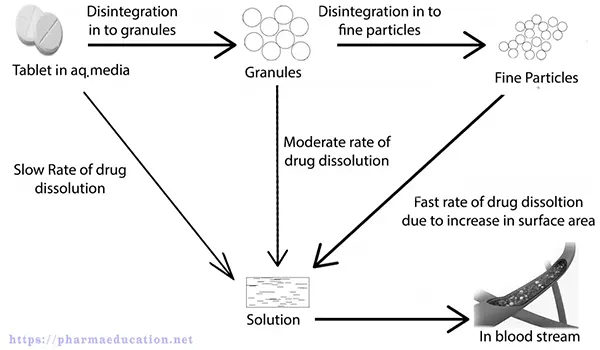
Probably, disintegrants act by the following one or more mechanisms:
- By Swelling
- Due to the heat of wetting
- Capillary action (wicking)
- Enzymatic reaction
- Due to the release of gases
- Combination action
- Deformation
- Electrostatic repulsion
- Chemical reaction
List of Disintegrants Used in pharmaceutical preparations
| Name of Disintegrants | Concentration |
| 1. Calcium Alginate & Calcium Sodium Alginate | <10% |
| 2. Calcium carboxymethylcellulose / calcium cellulose glycolate / carmellose calcium | 1–15% |
| 3. Microcrystalline Cellulose (MCC) | 5–15% |
| 4. Powdered Cellulose | 5–20% |
| 5. Chitosan Hydrochloride | —- |
| 6. Corn Starch and Pregelatinized Starch | —- |
| 7. Crospovidone (commercial name- Kollidon) | 2–5% |
| 8. Docusate Sodium | ≈ 0.5% |
| 9. Low-Substituted Hydroxypropyl Cellulose | —- |
| 10. Hydroxypropyl Starch | —- |
| 11. Magnesium Aluminum Silicate | 2-10% |
| 12. Methylcellulose | 2.0–10.0% |
| 13. Sodium Alginate | 2.5–10% |
| 14. Starch | 3–25% w/w |
| 15. Pregelatinised Starch | 5–10% |
| 16. Sodium Starch Glycolate (Commercial name Primogel, Explotab) | 2-8%, Optimum concentration is about 4%, although 2% is sufficient in many cases. |
| 17. Croscarmellose Sodium (Commercial name Ac-Di-Sol) | 10–25% in capsules and 0.5–5.0% in tablets. Normally, Croscarmellose sodium at concentrations up to 5% w/w may be used as a tablet disintegrant. 2% w/w is used in direct compressed tablets and 3% w/w in wet-granulation processed tablets. |
Why disintegrants are added before and after granulation frequently?
In many tablet formulations, disintegrants are added before and after wet granulation or dry granulation. When disintegrants come in contact with a liquid medium in a beaker of a disintegration apparatus or gastrointestinal fluid, they absorbed the liquid and start to swell, dissolve, or form gels. This causes the tablet structure to rupture and disintegrate, making increased surfaces for improved dissolution of the drug substance. Disintegrants are added after granulation to fast disintegrate the whole tablet into small granules. Also, Disintegrants are added before granulation to rapidly disintegrate the small granules into single particles of API and excipients. for improved dissolution of the drug substance.
Functions of Disintegrants in pharmaceutical dosage forms
- To promote rapid disintegration or break down of oral solid dosage form into small particles after administration for facilitating rapid dissolution into GI fluid.
- Added to an oral solid dosage form to get quicker drug release.
- They are used to control the disintegration time of oral solid dosage forms such as tablets, capsules, pellets, and granules as per pharmacopeia.
Generally, disintegrants are not required for any non-oral solid, liquid, semisolid, or parenteral pharmaceutical preparations.
Disintegration Time Test
Disintegration is the process by which a solid oral dosage form such as a tablet breaks down into smaller particles or granules. The tablets must disintegrate and all particles must pass through the 10-mesh screen in the time specified. Complete disintegration is that state in which any residue of the unit (tablet or capsule or granules) except fragments of insoluble coating or capsule shell, remaining on the screen of the disintegration apparatus or adhering to the lower surface of the disk if used, is a soft mass having no palpably firm core. Disintegration is provided to determine whether tablets, capsules, or granules disintegrate within the prescribed time when placed in a suitable liquid medium in a 1000 ml beaker at 37°C ± 2°C. It is a pharmacopoeial test for the evaluation of tablets or quality control tests of tablets.
References
- Rowe, R. C., Sheskey, P. J., Owen, S. C., & American Pharmacists Association. (2006). Handbook of pharmaceutical excipients. London: Pharmaceutical Press.
- British Pharmacopoeia Commission. British Pharmacopoeia 2021. London: TSO.
- The United States pharmacopeia The National formulary. Rockville, Md.: United States Pharmacopeial Convention, Inc. (USP 21 – NF 16).
- Lachman, Lieberman, H.A. and Kanig, J.L., The Theory and Practice of Industrial Pharmacy, Lea and Febiger, New York, 15th edition; 2013
You may also read
- DIFFERENCE BETWEEN BLISTER PACKAGING AND STRIP PACKAGING
- DIFFERENCE BETWEEN LUBRICANT AND GLIDANT
- Sweetening agents used in Pharmaceutical Preparations
- Emulsifying Agents (Emulsifiers or Emulgents) Used in Pharmaceuticals
- Tablet defects or manufacturing defects of tablet
- Quality control tests of tablets or evaluation of tablets